Pentaerythritol
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
2,2-Bis(hydroxymethyl)propane-1,3-diol[1] | |
| Other names
2,2-Bis(hydroxymethyl)1,3-propanediol
Pentaerythritol[1] Hercules P 6 Monopentaerythritol Tetramethylolmethane THME PETP Pentaerythrite Pentek Hercules Aqualon improved technical PE-200 | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.732 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C5H12O4 | |
| Molar mass | 136.15 g/mol |
| Appearance | white solid |
| Density | 1.396 g/cm3 |
| Melting point | 260.5 °C (500.9 °F; 533.6 K) |
| Boiling point | 276 °C (529 °F; 549 K) at 30 mmHg |
| |
| Solubility |
Slightly soluble in:methanol, ethanol, glycerol, ethylene glycol, formamide; insoluble in: acetone, toluene, heptane, diethyl ether, dichloromethane |
| Vapor pressure | 0.00000008 mmHg (20°C)[4] |
| Hazards | |
| Flash point | 200.1 °C (392.2 °F; 473.2 K) |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp)[4] |
REL (Recommended)
|
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp)[4] |
IDLH (Immediate danger)
|
N.D.[4] |
| Related compounds | |
Related compounds
|
Neopentane, Neopentyl alcohol, Neopentyl glycol, Trimethylolethane, Orthocarbonic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
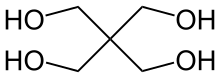
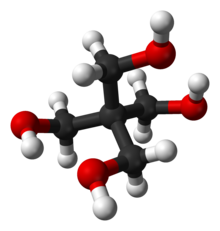
Pentaerythritol is an organic compound with the formula C(CH2OH)4. The molecular structure can be described as a neopentane with one hydrogen atom in each methyl group replaced by a hydroxyl (–OH) group. It is therefore a polyol, specifically a tetrol.
Pentaerythritol is a white solid. It is a building block for the synthesis and production of explosives, plastics, paints, appliances, cosmetics, and many other commercial products.
The word pentaerythritol is a blend of penta- in reference to its five carbon atoms and erythritol, which also possesses 4 alcohol groups.
Synthesis
[edit]Pentaerythritol was first reported in 1891 by German chemist Bernhard Tollens and his student P. Wigand.[5] It may be prepared via a base-catalyzed multiple-addition reaction between acetaldehyde and 3 equivalents of formaldehyde to give pentaerythrose (CAS: 3818-32-4), followed by a Cannizzaro reaction with a fourth equivalent of formaldehyde to give the final product plus formate ion.[6]
Uses
[edit]Pentaerythritol is a versatile building block for the preparation of many compounds,[7] particularly polyfunctionalized derivatives. applications include alkyd resins, varnishes, polyvinyl chloride stabilizers, tall oil esters, antioxidants (e.g. Anox 20). Such derivatives are found in plastics, paints, cosmetics, and many other products.[8]
Esters of pentaerythitol are biodegradable,[9][10] and they are used as transformer oils.[11] Due to a very high flash point they also find some use in lubricating gas turbines.[12]
Ester derivatives
[edit]Pentaerythritol is a precursor to esters of the type C(CH2OX)4. Such derivatives are pentaerythritol tetranitrate (PETN), a vasodilator and explosive, the trinitrate derivative pentrinitrol (Petrin), the tetraacetate normosterol (PAG), and the polymer cross-linking agents pentaerythritol tetraacrylate and pentaerythritol tetrakis(3-mercaptopropionate).[13][14]
A linear polymer which can be described as a (spiro) orthocarbonate ester of pentaerythritol, whose formula could be written as [(−CH2)2C(CH2−)2 (−O)2C(O−)2]n, was synthesized in 2002.[15]
Fire retardants
[edit]Pentaerythritol is used as a fire retardant, such as in plastics and intumescent paints and coatings. It releases water upon heating and leaves a deposit of thermally insulating char.[16]
See also
[edit]References
[edit]- ^ a b Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 691. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ Yalkowsky, Samuel H. (2010). Handbook of aqueous solubility data (Second ed.). Boca Raton, FL: CRC Press. p. 185. ISBN 9781439802465.
- ^ Yadav, Manish G.; Vadgama, Rajeshkumar N.; Kavadia, Monali R.; Odaneth, Annamma Anil; Lali, Arvind M. (September 2019). "Production of Pentaerythritol Monoricinoleate (PEMR) by immobilized Candida antarctica lipase B". Biotechnology Reports. 23: e00353. doi:10.1016/j.btre.2019.e00353. PMC 6599945. PMID 31304100.
- ^ a b c d NIOSH Pocket Guide to Chemical Hazards. "#0485". National Institute for Occupational Safety and Health (NIOSH).
- ^ Tollens, B.; Wigand, P. (1891). "Ueber den Penta-Erythrit, einen aus Formaldehyd und Acetaldehyd synthetisch hergestellten vierwerthigen Alkohol (On pentaerythritol, a quaternary alcohol synthetically produced from formaldehyde and acetaldehyde)" (PDF). Justus Liebig's Annalen der Chemie (in German). 265 (3): 316–340. doi:10.1002/jlac.18912650303.
- ^ Schurink, H. B. J. (1925). "Pentaerythritol". Organic Syntheses. 4: 53. doi:10.15227/orgsyn.004.0053; Collected Volumes, vol. 1, p. 425.
- ^ Marrian, S. F. (1 August 1948). "The Chemical Reactions of Pentaerythritol and its Derivatives". Chemical Reviews. 43 (1): 149–202. doi:10.1021/cr60134a004. PMID 18876970.
- ^ Werle, Peter; Morawietz, Marcus; Lundmark, Stefan; Sörensen, Kent; Karvinen, Esko; Lehtonen, Juha (2008). "Alcohols, Polyhydric". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_305.pub2. ISBN 978-3527306732.
- ^ NPCS Board of Consultants & Engineers (2016). The Complete Book on Adhesives, Glues & Resins Technology (with Process & Formulations) 2nd Revised Edition.
- ^ NIIR Board of Engineers & Consultants (2005). Synthetic Resins Technology Handbook.
- ^ Rudnick, Leslie R. (22 December 2005). Synthetics, Mineral Oils, and Bio-Based Lubricants: Chemistry and Technology. ISBN 9781420027181.
- ^ Bhushan, Bharat (28 December 2000). Modern Tribology Handbook, Two Volume Set. ISBN 9780849377877.
- ^ S. F. Marrian (1948). "The Chemical Reactions of Pentaerythritol and its Derivatives". Chemical Reviews. 43 (1): 149–202. doi:10.1021/cr60134a004. PMID 18876970.
- ^ Hoyle, Charles E.; Bowman, Christopher N. (2010). "Thiol–Ene Click Chemistry". Angewandte Chemie International Edition. 49 (9): 1542–1543. doi:10.1002/anie.200903924.
- ^ David T. Vodak, Matthew Braun, Lykourgos Iordanidis, Jacques Plévert, Michael Stevens, Larry Beck, John C. H. Spence, Michael O'Keeffe, Omar M. Yaghi (2002): "One-Step Synthesis and Structure of an Oligo(spiro-orthocarbonate)". Journal of the American Chemical Society, volume 124, issue 18, pages 4942–4943. doi:10.1021/ja017683i
- ^ Stoye, Dieter; et al. (2006). "Paints and Coatings". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a18_359.pub2. ISBN 3527306730.

